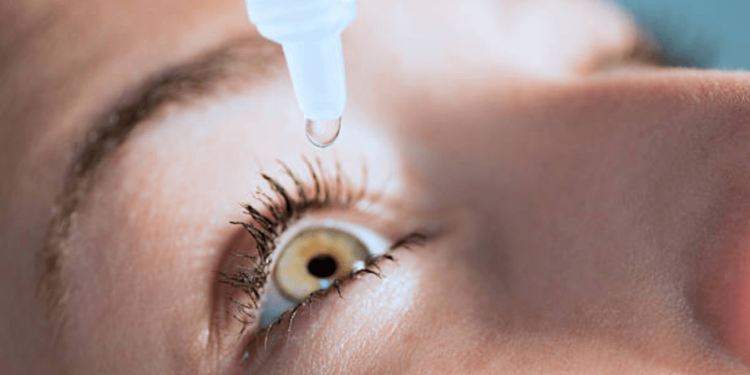
The Food and Drug Administration (FDA) warned the public this week against purchasing three brands of contaminated copycat eye drops due to the risk of potential infection.
In an announcement released Wednesday, the FDA strongly advised against purchasing or utilizing South Moon, Rebright and FivFivGo eye drops, as the brands are not approved to ease eye redness and could be mistaken for the Bausch + Lomb Lumify drops.
“South Moon, Rebright and FivFivGo eye drops are unapproved drugs and should not be available for sale in the U.S.,” the agency wrote in its release. “They claim to treat eye conditions such as glaucoma, which is treated with prescription drugs or surgery.”
In its testing, the FDA found that South Moon samples carried the Burkholderia cepacia complex, a bacteria group that could lead to an antibiotic-resistant infection.
Rebright did not have any contamination. However, both South Moon and Rebright lacked brimonidine tartrate, the active ingredient in Lumify, which has been approved by the FDA.
While the agency could not obtain samples of FivFivGo, officials encouraged the public against buying and using the product.
The FDA is still investigating the origin of the unapproved products. Consumers should look for medical care if they have symptoms of an eye infection after using the copycat drops, according to the FDA. They also warned that consumers should be careful when buying eye products online and stick to drops from licensed pharmacies.
Last November, the FDA warned against using 26 eye drop products made by multiple major brands due to the risk of eye infections that could end up causing loss of vision or blindness.
In February 2023, the administration widened the advisory on some eye products, claiming some could lead to hospitalization or blindness.