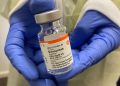
The Supreme Court ruled in a unanimous decision Thursday that a group of anti-abortion doctors does not have any legal basis to challenge access to mifepristone, one of the two common drugs used in medication abortion.
As a result, access to mifepristone won’t change.
The opinion, written by Justice Brett Kavanaugh, sided with the Biden administration and Danco, the manufacturer of the branded version of mifepristone. It reversed a lower court decision that would have made it more difficult to obtain the drug, which is used in about two-thirds of U.S. abortions.
The ruling didn’t address the underlying regulatory or safety issues the plaintiffs raised, instead deciding the case only on standing. The justices found the conservative doctors in the lawsuit did not show they had personally been harmed by the government’s actions regulating mifepristone.
“Under Article III of the Constitution, a plaintiff’s desire to make a drug less available for others does not establish standing to sue,” Kavanaugh wrote.
The drug will remain available to people up to the 10th week of pregnancy and will still be available through the mail. The decision is a victory for the Biden administration’s efforts to maintain access to abortion, but it also is a victory for the Food and Drug Administration’s (FDA) authority to regulate drugs.
Leading up oral arguments, pharmaceutical companies and FDA law experts urged the court not to second-guess the agency’s expertise and side with the plaintiffs. A ruling against the administration could have undermined the entire drug approval process, they argued.
“We are pleased with the Supreme Court’s decision in this incredibly important case. By rejecting the Fifth Circuit’s radical, unprecedented and unsupportable interpretation of who has standing to sue, the justices reaffirmed longstanding basic principles of administrative law,” said Abigail Long, a spokesperson for Danco. “The decision also safeguards access to a drug that has decades of safe and effective use.”
Still, mifepristone remains illegal in the more than a dozen states that ban abortion.
The case centered on whether federal regulators overstepped their authority by loosening restrictions to make mifepristone easier to access. The FDA first approved mifepristone in 2000 for abortion up to seven weeks of pregnancy, but then made a series of changes in 2016 and 2021.
Those changes included increasing the gestational age at which mifepristone can be used to up to 10 weeks of pregnancy, allowing the medication to be mailed to patients, lowering the dosage, allowing telehealth prescribing, and permitting providers other than physicians to prescribe the drug.
The FDA has repeatedly found that mifepristone is safe and that a medication abortion regimen that includes mifepristone and a second drug, misoprostol, is a safe and effective alternative to surgical abortions.
But anti-abortion groups contend the drugs are dangerous. A coalition of anti-abortion doctors and associations sued the administration, and a federal district judge in Texas ruled in their favor, invalidating the drug’s entire approval.
The Biden administration appealed, and a ruling from the U.S. Court of Appeals for the 5th Circuit found the drug was legally approved but that the FDA’s actions to loosen restrictions were not constitutional.
At oral arguments before the Supreme Court in March, the doctors contended that as a direct result of the FDA’s actions, pregnant women will suffer complications from mifepristone, so they may be required at some point — against their consciences — to render emergency treatment completing an abortion or providing other abortion-related treatment.
But Kavanaugh wrote that federal conscience protection laws “definitively protect doctors from being required to perform abortions or to provide other treatment that violates their consciences.”
Since the Supreme Court eliminated the nationwide right to abortion in 2022, more people have been using medication to terminate pregnancies. Medication abortion accounted for 63 percent of all abortions in the formal health care system in 2023, according to the Guttmacher Institute, an abortion-rights research group.
Abortion-rights advocates breathed a sigh of relief but noted the case won’t be the last time abortion access is challenged.
“This case should never have made it to the Supreme Court in the first place. Anti-abortion operatives brought this case with one goal in mind – to ban medication abortion and they failed. This case was a near miss for the science and medicine community and it won’t be the last attack,” said Haydee Morales, interim president of the National Institute for Reproductive Health.
“This should be a warning to all of us: if anti-abortion operatives came this close to undermining long-standing approvals for mifepristone, what will come next?” Morales added.
Anti-abortion groups have indicated the decision was only a temporary setback, and they are confident they can find another way to challenge the drugs.
For instance, the same district court in Texas that originally ruled against the FDA said a group of red states led by Missouri can intervene in the lawsuit.
Updated at 10:55 a.m.